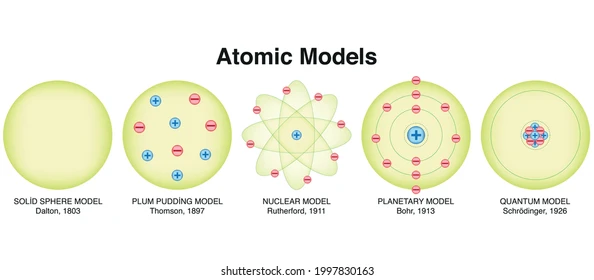
Atomic Models
Solid Sphere Model
The solid sphere model, also known as Dalton’s atomic theory, was the first atomic model proposed by John Dalton in 1803. It posits that all matter is composed of tiny, indivisible particles called atoms, which are solid, indestructible spheres. Different elements are made of atoms with different masses and properties, and atoms combine in simple whole-number ratios to form compounds.
Although Dalton’s model was a significant step forward in understanding the atom, it was later refined and expanded upon by subsequent scientists and models. It did not account for the existence of subatomic particles (electrons, protons, and neutrons) or the internal structure of the atom. Later models, such as the plum pudding model and Rutherford’s nuclear model, addressed these limitations.
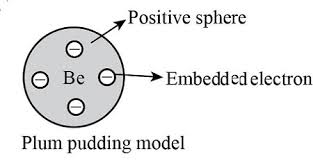
Plum Pudding Model
The plum pudding model, also known as the Thomson model, was an early atomic model proposed by J.J. Thomson. It described the atom as a sphere of positive charge with negatively charged electrons embedded within it, like plums in a pudding. This model was developed after Thomson’s discovery of the electron and aimed to explain the atom’s overall neutrality.
This depicted the atom as a sphere of positive charge with negatively charged electrons embedded within it, like plums in a pudding.
This early model, also known as the Thomson model, was an attempt to explain the atom’s neutral charge, suggesting that the positive charge balanced the negative charge of the electrons.
While the plum pudding model was a significant step in understanding atomic structure, it was later superseded by the Rutherford model after the gold foil experiment demonstrated the existence of a nucleus.
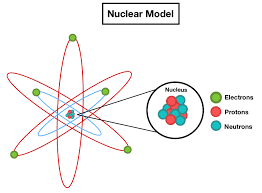
Nuclear Model
The nuclear model of the atom, developed by Ernest Rutherford, describes the atom as having a positively charged nucleus at its center, where nearly all of the atom’s mass is concentrated, surrounded by a cloud of negatively charged electrons. This model revolutionized our understanding of atomic structure, moving away from the earlier plum pudding model.
Rutherford’s model is called the nuclear model because it was the first to propose a central nucleus within the atom. This nucleus, containing most of the atom’s mass and positive charge, is surrounded by orbiting electrons. The discovery of this nucleus, through the gold foil experiment, fundamentally changed the understanding of atomic structure.
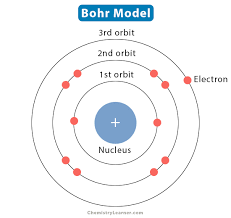
Planetary Model
The term “planetary model” can refer to two distinct concepts: a simplified model of the atom and a physical model of the solar system. In the context of atomic structure, it describes the Bohr model, where electrons orbit the nucleus in specific paths, similar to planets around the sun. In the context of the solar system, it refers to physical models that depict the relative positions and motions of planets.
While the Bohr model successfully explained the hydrogen spectrum and introduced the concept of quantized energy levels, it was later superseded by more sophisticated quantum mechanical models that provide a more accurate representation of atomic structure.
Quantum Model
The quantum mechanical model (or quantum model) of the atom is the most accurate and advanced theory describing the behavior of electrons in atoms. It departs from earlier models by portraying electrons not as particles in fixed orbits, but as waves occupying three-dimensional regions called orbitals, described by probability distributions. This model incorporates principles like wave-particle duality and the Heisenberg uncertainty principle.
In essence, the quantum model provides a probabilistic framework for understanding the behavior of electrons within an atom, moving beyond the deterministic picture of electrons in fixed orbits to a more nuanced view of their wave-like nature and spatial distribution.